Mounjaro 10 mg Pen (Tirzepatide) | Diabetes & Weight Loss | Lilly
Mounjaro 10 mg is a key maintenance dose of the prescription, once-weekly injectable medication containing the active ingredient Tirzepatide. Manufactured by Lilly, Mounjaro is a breakthrough first-in-class treatment approved for adults with type 2 diabetes to improve blood sugar control. It is also highly effective for weight management.
Details
Description
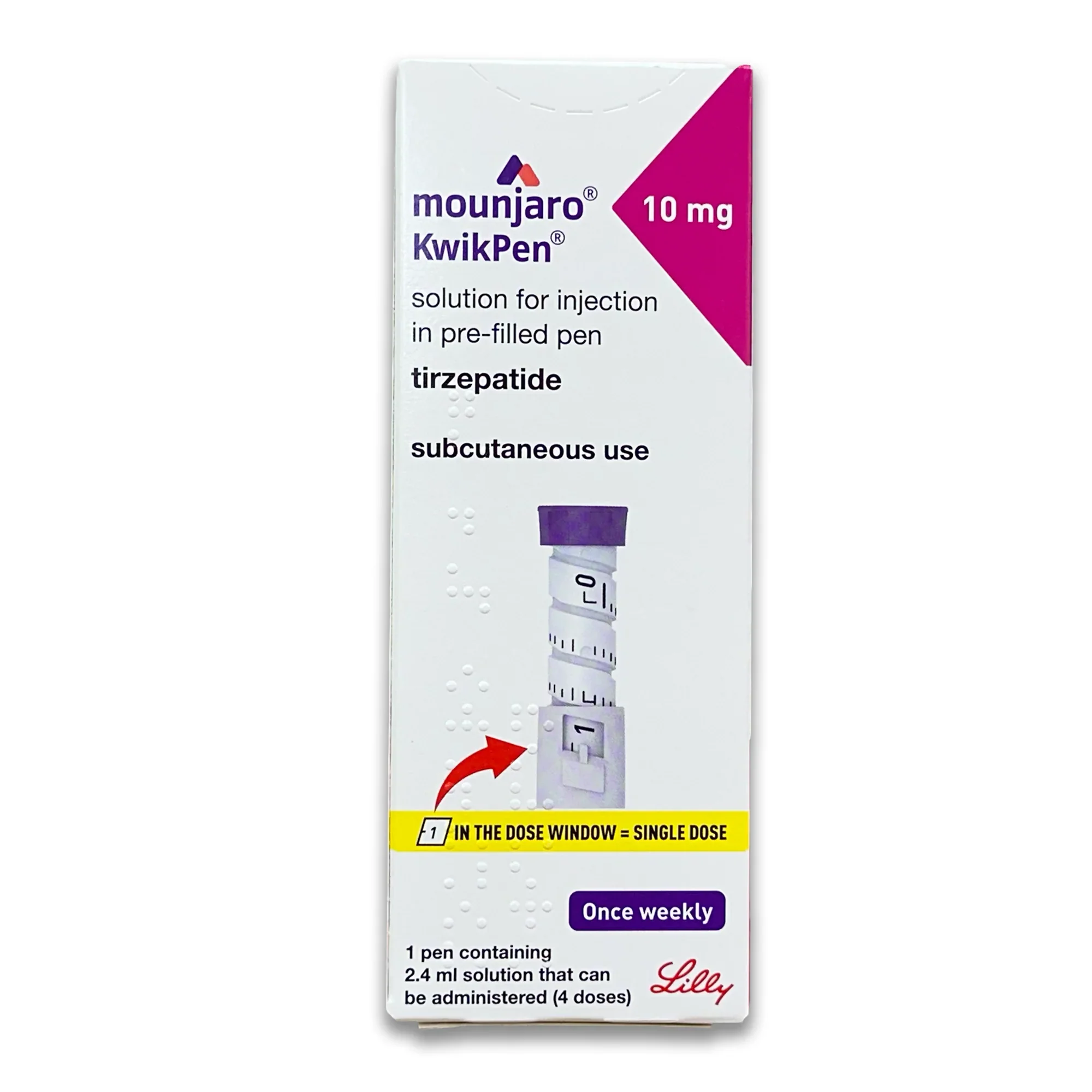
Mounjaro 10 mg (Tirzepatide) Pen – 4 Pack
Mounjaro 10 mg is a key maintenance dose of the prescription, once-weekly injectable medication containing the active ingredient Tirzepatide. Manufactured by Lilly, Mounjaro is a breakthrough first-in-class treatment approved for adults with type 2 diabetes to improve blood sugar control. It is also highly effective for weight management.
Product Overview: Mounjaro 10 mg (Tirzepatide)
Mounjaro (Tirzepatide) is a unique medication. It is the first and only dual-action GIP and GLP-1 receptor agonist. This dual mechanism targets two key hormones that regulate blood sugar and appetite.
The 10 mg dose is a strong maintenance dose. It is prescribed to patients who have already completed the initial dose-escalation steps (e.g., 2.5 mg, 5 mg, 7.5 mg) and require further blood sugar control and support for weight loss.
This package contains 4 single-dose, pre-filled pens, providing a 4-week supply.
How Does Mounjaro (Tirzepatide) Work?
The active ingredient, Tirzepatide, works by activating two separate hormone receptors:
- GLP-1 (Glucagon-like peptide-1): This helps stimulate insulin release, reduce sugar production from the liver, and slow down digestion, making you feel full.
- GIP (Glucose-dependent insulinotropic polypeptide): This also enhances insulin release and is believed to play a role in fat metabolism and appetite regulation.
By targeting both pathways, Mounjaro provides powerful control over blood sugar (A1C) and appetite, leading to significant weight loss in many patients.
Key Product Details
| Feature | Detail |
| Brand: | Mounjaro |
| Manufacturer: | Lilly |
| Active Ingredient: | Tirzepatide |
| Dosage Strength: | 10 mg (Maintenance Dose) |
| Form: | Solution for Injection (Single-dose Pen) |
| Package Size: | 4 x 10 mg single-dose pens |
| Indication: | Type 2 Diabetes; Weight Management |
Dosage and Administration
Mounjaro is a once-weekly subcutaneous injection (an injection under the skin) that you can administer yourself.
- Maintenance Dose: The 10 mg dose is a maintenance dose, taken once per week.
- Titration: This dose is only used after a patient has successfully completed at least 4 weeks on the 7.5 mg dose. Your doctor may then increase the dose to 12.5 mg or 15 mg if needed.
Important: This is a prescription-only medication. You must follow the exact titration and dosing schedule prescribed by your healthcare provider. Do not start with this dose.
Frequently Asked Questions (FAQ) about Mounjaro 10 mg
Is 10 mg a high dose for Mounjaro?
Yes, 10 mg is considered a strong maintenance dose. It provides significant glycemic control and weight loss. The dose is titrated up from 2.5 mg, 5 mg, and 7.5 mg. The maximum dose is 15 mg.
What is the difference between Mounjaro (Tirzepatide) and Ozempic (Semaglutide)?
Ozempic (Semaglutide) is a GLP-1 receptor agonist. Mounjaro (Tirzepatide) is a dual-action GIP and GLP-1 receptor agonist. This dual mechanism is unique to Mounjaro and is why it has shown different results in clinical studies.
How must Mounjaro pens be stored?
- Unopened Pens: Must be stored in a refrigerator (between 2°C and 8°C or 36°F and 46°F).
- In-Use Pens: If needed, a single pen can be stored at room temperature (below 30°C or 86°F) for up to 21 days.
- Do not freeze Mounjaro.
What are the most common side effects of Mounjaro?
The most common side effects are gastrointestinal. These include nausea, diarrhea, decreased appetite, vomiting, and constipation. These side effects are most common when starting or increasing the dose, and often lessen over time.
Important Safety Information
WARNING: RISK OF THYROID C-CELL TUMORS
- Tirzepatide (Mounjaro) has been shown to cause thyroid C-cell tumors in animal studies. It is not known if Mounjaro causes these tumors in humans.
- Do not use Mounjaro if you or any of your family members have ever had Medullary Thyroid Carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Before taking Mounjaro, tell your doctor about your full medical history, especially if you have:
- A history of pancreatitis (inflammation of the pancreas).
- Kidney problems or diabetic retinopathy.
- Severe gastrointestinal (stomach) problems.
- Are pregnant, planning to become pregnant, or breastfeeding.
Disclaimer: This content is for informational purposes only and is not a substitute for professional medical advice. Mounjaro (Tirzepatide) is a prescription medication with serious risks. Always consult with your healthcare provider to see if Mounjaro is right for you.




Reviews
Clear filtersThere are no reviews yet.